Safety Notifications and reporting pathways for therapeutic goods trials
sourced: https://www.medicalresearch.nsw.gov.au/clinical-trial-safety-monitoring/ - Sept. 2023
Therapeutic Goods Trials: Trials investigating the safety and/or effectiveness of medicines, biologicals or medical devices.
Non-Therapeutic Goods Trials: Trials other than a Therapeutic Goods Trial (e.g. radiotherapy, surgery, psychotherapy trials).
Suspected Unexpected Serious Adverse Reaction (SUSAR): An adverse reaction that is both serious and unexpected.
Unanticipated Serious Adverse Device Effects (USADEs): A serious adverse device effect which by its nature, incidence, severity or outcome has not been identified in the current version of the risk analysis report (and/or Investigator’s Brochure/Instructions for Use).
Urgent Safety Measure (USM): A measure required to be taken in order to eliminate an immediate hazard to a participant’s health or safety.
Significant Safety Issue (SSI): A safety issue that could adversely affect the safety of participants or materially impact on the continued ethical acceptability or conduct of the trial.
Unexpected & Related SAEs (URSAE): An adverse event that is:
- Serious – meets the definition of a serious adverse event
- Related – resulted from administration of the trial intervention
- Unexpected – the event is not described in the protocol as an expected occurrence.
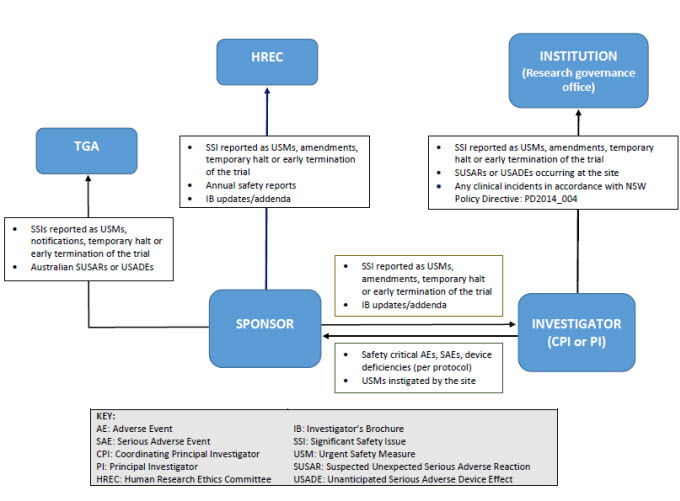
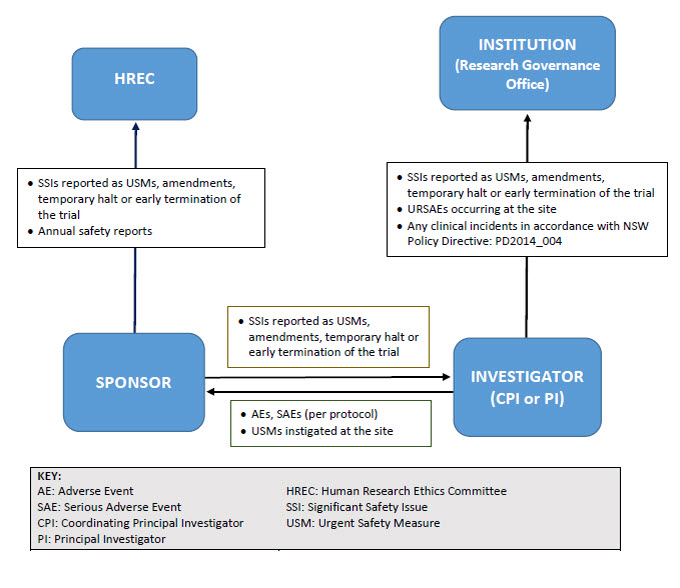
Human Research Ethics Committees will no longer receive single case AEs, SAE/SARs and SUSARs* or device/non-therapeutic good trial equivalents or six monthly line listings.
Human Research Ethics Committees will receive all significant safety issues, annual safety reports and investigator’s brochure updates.
Research Governance Offices will no longer receive single case AEs, SAE/SARs and external SUSARs* or device/non-therapeutic good equivalents or six monthly line listings.
Research Governance Offices will receive all significant safety issues (SSIs), any local SUSARs/USADEs/URSAEs and any research-related events that meet the definition of an incident (PD2014_004).
*Note: If a SAE/SAR/ SUSAR meets the definition of an SSI, it will be reported to the HREC/RGO through that reporting mechanism.
Summary Table
|
Type of event |
Who reports |
To whom |
When | How |
|---|---|---|---|---|
|
Significant Safety Issue (SSI) implemented as an Urgent Safety Measure (USM) |
Sponsor/Delegate |
The reviewing HREC (and all investigators participating in the study) |
As soon as possible and no later than 72 hours of the sponsor becoming aware of the USM |
SSI Notification Form; Sponsor’s template |
|
Significant Safety Issue (SSI) not implemented as an Urgent Safety Measure (USM) |
Sponsor/Delegate |
The reviewing HREC (and all investigators participating in the study) |
Within 15 days of the sponsor becoming aware of the SSI |
SSI Notification Form; Sponsor’s template |
|
All Significant Safety Issues (SSIs) |
Principal Investigator |
The RGO for the site where the event occurred |
As soon as possible and no later than 72 hours of the PI becoming aware of the SSI |
SSI Notification Form; Sponsor’s template |
|
Suspected Unexpected Serious Adverse Events (SUSARs) and Unanticipated Serious Adverse Device Effects (USADEs) occurring at the site |
Principal Investigator |
The RGO for the site where the event occurred |
Within 72 hours of the PI becoming aware of the event |
SUSAR/USADE/URSAE Notification Form |
|
Investigator’s Brochure Updates/Addenda |
Sponsor/Delegate |
The reviewing HREC |
As and when updates are generated |
Submitted with a cover sheet or as part of an annual progress/annual safety report |
|
Annual Safety Report |
Coordinating Principal Investigator or Sponsor/Delegate |
The reviewing HREC |
Within annual progress report sent to the HREC or aligned with the safety reporting cycles of global companies |
Annual Progress Report or sponsor’s template |
As illustrated below, sponsors may report directly to NSW Human Research Ethics Committee; however, they must ensure that all communications sent to the Human Research Ethics Committee adequately identify the trial and provide context in relation to the Human Research Ethics Committee’s role (e.g. whether there is any impact on patient safety, trial conduct or trial documentation).
|
Type of event |
Who reports |
To whom |
When | How |
|---|---|---|---|---|
|
Significant Safety Issue (SSI) implemented as an Urgent Safety Measure (USM) |
Sponsor/Delegate |
The reviewing HREC (and all investigators participating in the study) |
As soon as possible and no later than 72 hours of the sponsor becoming aware of the USM |
SSI Notification Form; Sponsor’s template |
|
Significant Safety Issue (SSI) not implemented as an Urgent Safety Measure (USM) |
Sponsor/Delegate |
The reviewing HREC (and all investigators participating in the study) |
Within 15 days of the sponsor becoming aware of the SSI |
SSI Notification Form; Sponsor’s template |
|
All Significant Safety Issues (SSIs) |
Principal Investigator |
The RGO for the site where the event occurred |
As soon as possible and no later than 72 hours of the PI becoming aware of the SSI |
SSI Notification Form; Sponsor’s template |
|
Unexpected & Related Serious Adverse Event (URSAEs) occurring at the site |
Principal Investigator |
The RGO for the site where the event occurred |
Within 72 hours of the PI becoming aware of the event |
SUSAR/USADE/URSAE Notification Form |
|
Annual Safety Report |
Coordinating Principal Investigator or Sponsor/Delegate |
The reviewing HREC |
Annually (within the annual progress report) |
Annual Progress Report |
For further information Facts & Questions: https://www.medicalresearch.nsw.gov.au/clinical-trial-safety-monitoring/
Please refer to the REGIS website
or refer to the quick reference guide